
Figure 2: Typical Compounds with Ionic, Polar Covalent, and Covalent Bonds

Covalent and Ionic Bonds

the creation of ionic bonds between two or more atoms in the molecule.

Day 44: Ionic & Covalent Bonds Venn Diagram October 18, 2006

Ionic - Covalent - Polar Covalent Bonds. Bond Types

Covalent Bonds

An ionic bond is formed when electrons are lost and gained by two or more

Illustrations of ionic bond, covalent bond and hydrogen bond.

Figure showing ionic and covalent bonding

In covalent bonds, pairs of electrons are shared between atoms in order to

Occurs in molecules that have covalent bonds. Sometimes the electrons are
!["PDF 10: Ionic and Covalent Bonds." [Podcast Entry] Science 7- Trimester Two "PDF 10: Ionic and Covalent Bonds." [Podcast Entry] Science 7- Trimester Two](http://upload.wikimedia.org/wikipedia/commons/d/d3/Sodium_bromide.jpg)
"PDF 10: Ionic and Covalent Bonds." [Podcast Entry] Science 7- Trimester Two

covalent bonds. In the same way and N-S orientation, the ionic molecules

Ionic bonds form when electrons are transferred. Covalent bonds form when

Example of ionic bonding: NaCl (Figure 2.8). F. Covalent bonding: atoms may

A. In ionic bonding, one atom either loses or gains electrons,
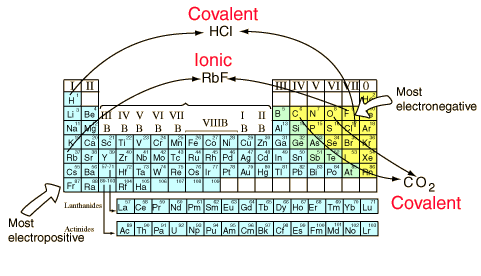
form covalent bonds and can exist as stable free molecules.
Ionic and covalent bonds. Introduction to ionic and covalent

Picture 2, -, Covalent and Ionic Bonds

When two non-metals join they form a covalent bond. The best that can happen
No comments:
Post a Comment